Introduction
Oxygen, symbolized by the atomic symbol O, is a highly significant element in the periodic table. It is the third most abundant element in the universe and plays a crucial role in various chemical reactions and biological processes. Understanding the valency of oxygen is essential for comprehending its chemical properties and relevance in different fields. In this article, we will delve into the concept of valency, explore the chemical properties of oxygen, and highlight its importance in both natural and industrial settings.
Table of Contents
- What is Valency?
- Understanding the Concept
- Valency of Elements
- The Valency of Oxygen
- Electron Configuration
- Octet Rule and Oxygen's Valency
- Common Valencies of Oxygen
- Chemical Properties of Oxygen
- Combustion and Oxidation Reactions
- Reaction with Metals
- Reaction with Non-Metals
- Formation of Oxides
- Relevance of Oxygen
- Role in Biological Systems
- Industrial Applications
- Conclusion
- FAQs
1. What is Valency?
1.1 Understanding the Concept
Valency refers to the combining capacity of an atom or an element with other atoms. It determines the number of bonds an atom can form with other elements to achieve a stable electron configuration. Valency is influenced by the number of valence electrons present in an atom.
1.2 Valency of Elements
Different elements exhibit various valencies based on their electronic structure. Valency can be categorized as monovalent, divalent, trivalent, and so on, depending on the number of electrons required to complete or stabilize the outermost electron shell.
2. The Valency of Oxygen
2.1 Electron Configuration
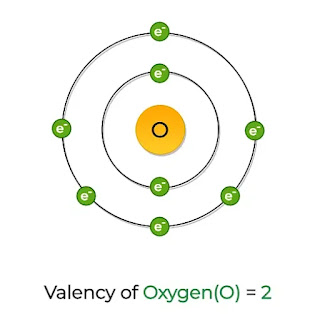
Oxygen has an atomic number of 8, indicating that it possesses eight protons and eight electrons in its neutral state. Its electron configuration is 1s^2 2s^2 2p^4, with six valence electrons in the outermost energy level.
2.2 Octet Rule and Oxygen's Valency
Oxygen follows the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration of eight valence electrons. Since oxygen requires two more electrons to complete its octet, it typically forms two covalent bonds.
2.3 Common Valencies of Oxygen
The most common valencies of oxygen are -2 and -1. In compounds, oxygen often gains two electrons (valency -2) or shares two electrons by forming a double bond (valency -1).
3. Chemical Properties of Oxygen
3.1 Combustion and Oxidation Reactions
Oxygen is highly reactive and readily participates in combustion and oxidation reactions. It supports combustion by reacting with other substances, such as carbon, to produce carbon dioxide and release energy. This property of oxygen makes it crucial for sustaining life through respiration and fueling various industrial processes.
3.2 Reaction with Metals
Oxygen reacts with most metals to form metal oxides. This reaction is known as oxidation. The formation of metal oxides can have diverse effects, ranging from rusting in iron to tarnishing in silver.
3.3 Reaction with Non-Metals
Oxygen also reacts with non-metals, such as sulfur and phosphorus, to form non-metal oxides. These reactions are significant in environmental processes, as they contribute to phenomena like acid rain and air pollution.
3.4 Formation of Oxides
Oxygen readily combines with other elements to form oxides, which are compounds containing oxygen atoms. Oxides have various applications, such as being essential components of ceramics, catalysts, and pigments.
4. Relevance of Oxygen
4.1 Role in Biological Systems
Oxygen plays a vital role in supporting aerobic respiration in living organisms. It acts as the final electron acceptor in the electron transport chain, facilitating the production of adenosine triphosphate (ATP) – the energy currency of cells. Additionally, oxygen is necessary for the sustenance of aquatic life, contributing to the oxygenation of water bodies.
4.2 Industrial Applications
Oxygen finds extensive applications in several industries. It is used in metal cutting and welding processes, where it reacts with metals at high temperatures to facilitate the cutting or shaping of metal surfaces. Oxygen is also employed in the production of steel, as it enhances the combustion of impurities and improves the efficiency of the steelmaking process.
5. Conclusion
In conclusion, understanding the valency of oxygen provides valuable insights into its chemical properties and relevance. Oxygen, with its versatile valency, exhibits a wide range of chemical reactions, including combustion, oxidation, and the formation of oxides. Its role in supporting life through respiration and its extensive applications in various industries make it an element of immense significance. By comprehending oxygen's valency, we can appreciate its fundamental role in numerous natural and industrial processes.
FAQs
- What is the valency of oxygen? Oxygen has a valency of -2 or -1, depending on the compound it forms.
- What are some chemical properties of oxygen? Oxygen is highly reactive, supporting combustion and oxidation reactions. It reacts with metals and non-metals to form oxides.
- What is the relevance of oxygen in biological systems? Oxygen is essential for aerobic respiration, acting as the final electron acceptor. It supports the production of ATP and contributes to the oxygenation of water bodies.
- How is oxygen used in industries? Oxygen is used in metal cutting, welding, and steelmaking processes. It enhances combustion and improves efficiency in various industrial applications.
- What is the octet rule? The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration of eight valence electrons.





0 Comments